- All atomic models showed only the electrons in the outermost shell.
- All chemical bonds are depicted as planar, but in reality, they can have three-dimensional structures.
- 'In case of unstable, display in red' may contain an error.
Chemical Bonding
Except for the noble gases of group 18, atoms are unstable and exist in a bonded state with other atoms. At this time, most atoms try to combine in a direction that forms the octet rule.
Octet Rule
The Octet Rule (or 8-electron rule) is an empirical rule in which atoms can exist in a stable state. In other words, each atom that makes up a molecule is in the most stable state when it has 8 electrons in its outermost shell.
However, there are some exceptions to the octet rule.
- The innermost shell is stable with two electrons. For example, hydrogen with atomic number 1 becomes stable when it gains one electron, and lithium with atomic number 3 becomes stable when it loses one electron. Number 2, helium, is the most stable atom in the world.
- From the 3rd period, substances appear that exist stably even if they do not satisfy the octet rule. (Example: H2SO4 (sulfuric acid), H3PO4 (phosphoric acid), etc.)
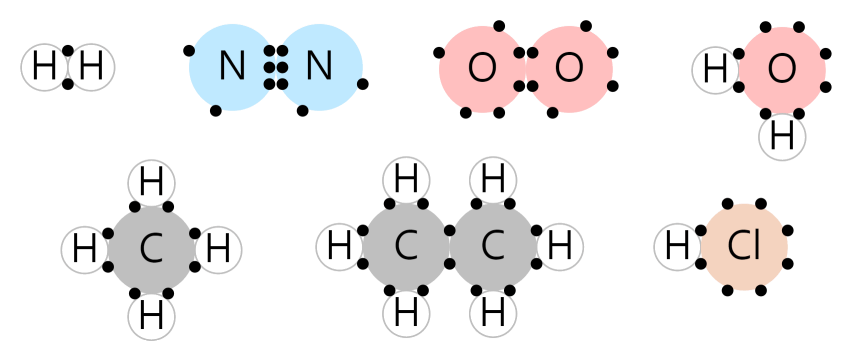
Covalent Bond
Atoms can share electrons to satisfy the octet rule.
The picture below is an example of a Covalent Bond. The electrons sandwiched between atoms are shared electrons. If we count each electron, including shared electrons, we see that hydrogen has two and the other atoms have 8.
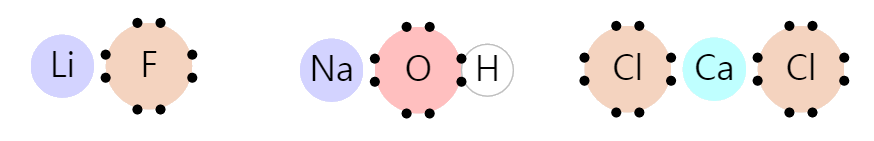
Ionic Bond
The ionic bond is a case where the octet rule is satisfied while donating electrons to another atom.
Five or more electrons must be collected to satisfy the octet rule if an atom has three or fewer outermost electrons. In this case, it is more advantageous to emit a few electrons instead of collecting many.
The picture below is an example of an Ionic Bond. Lithium (Li), sodium (Na), and calcium (Ca) give electrons to neighboring atoms. Lithium, sodium, and calcium lost their outermost electrons, and neighboring atoms satisfy the octet rule.