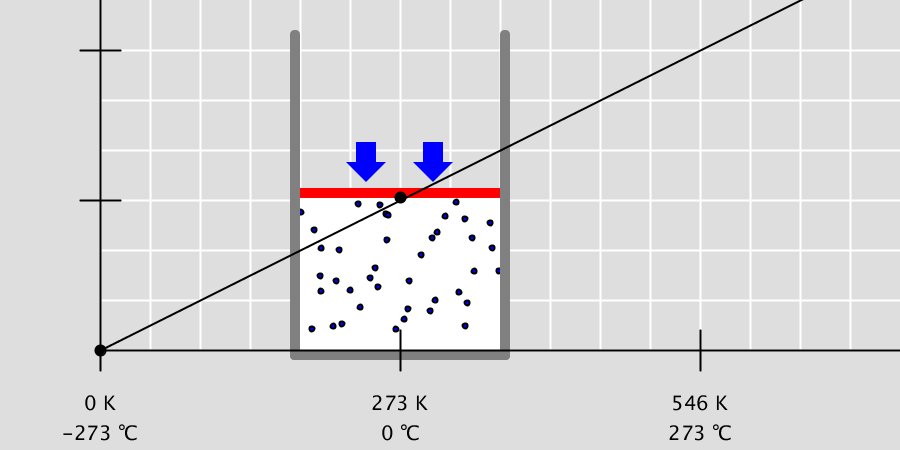
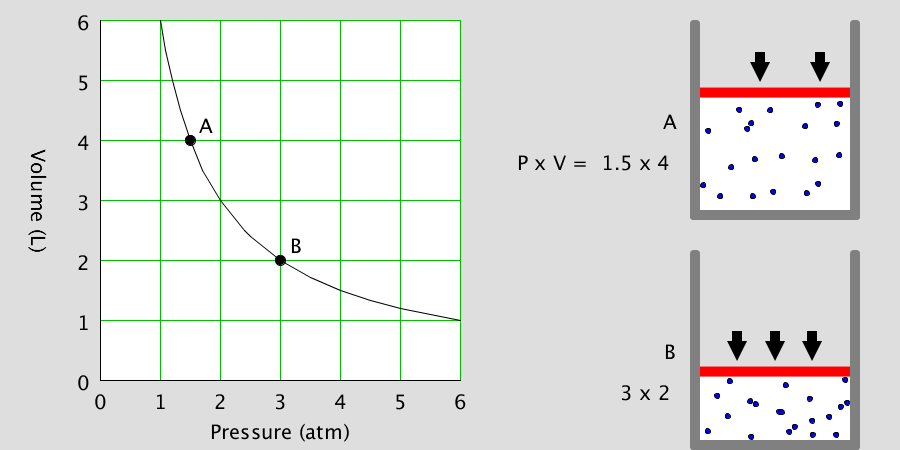
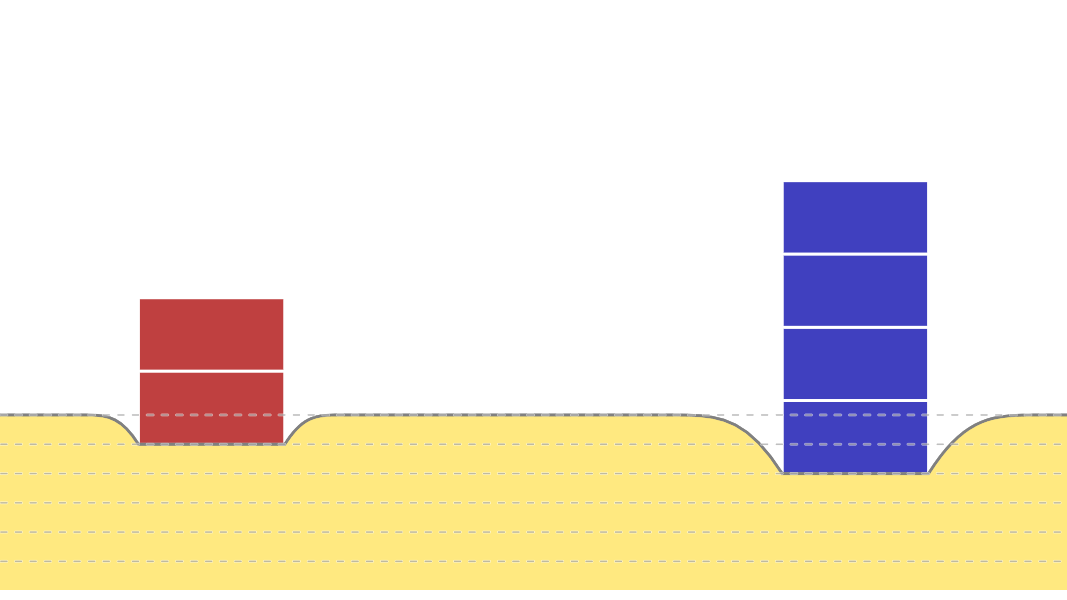
Kinetic Theory Model
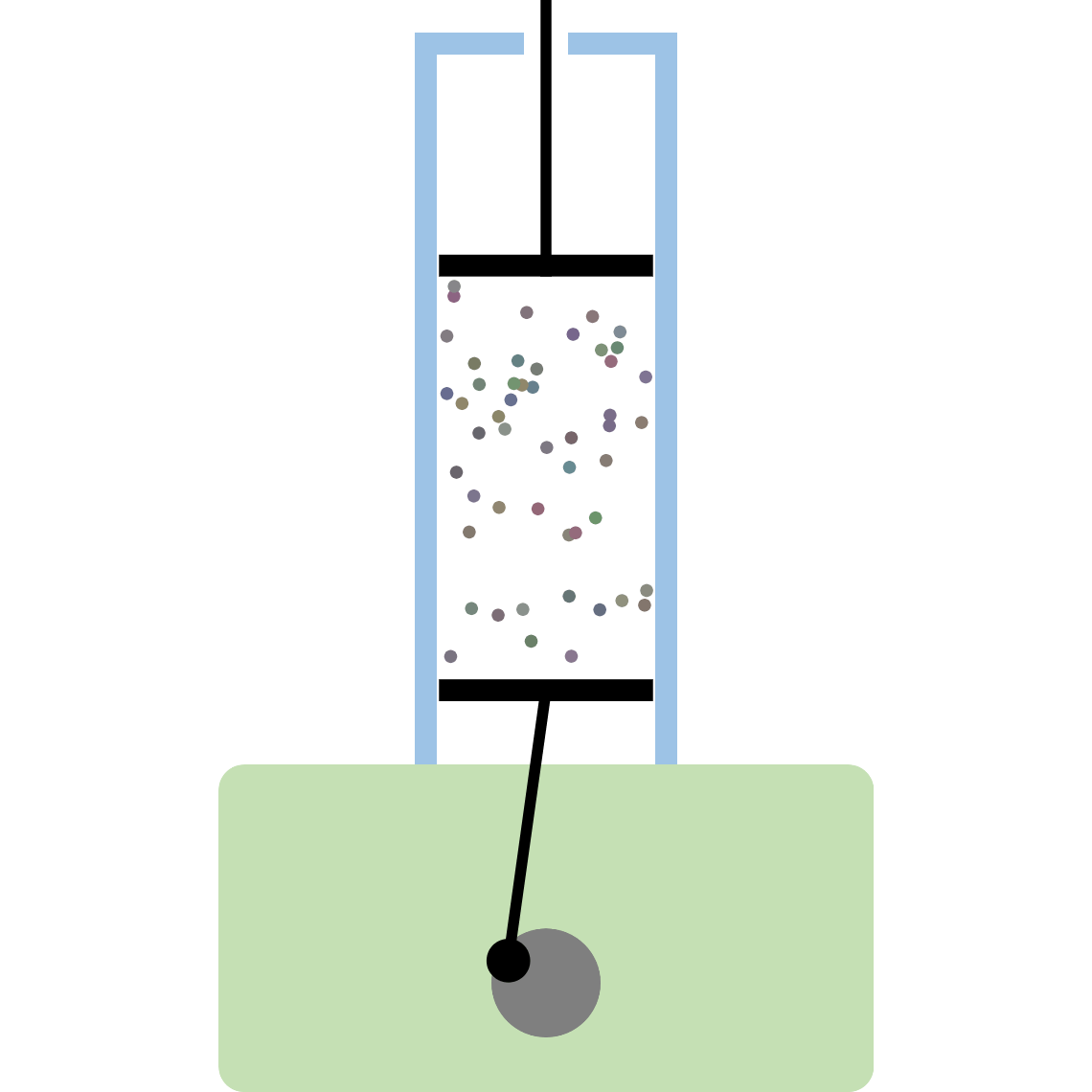
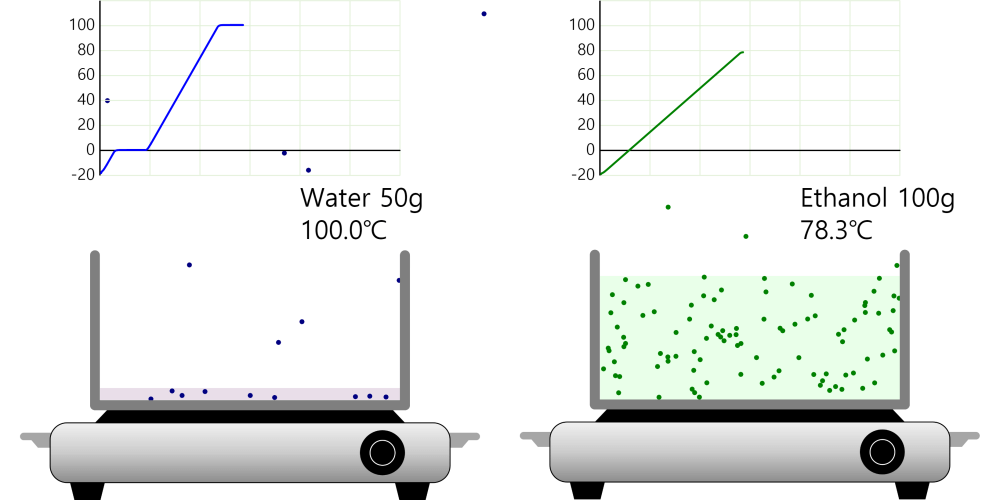
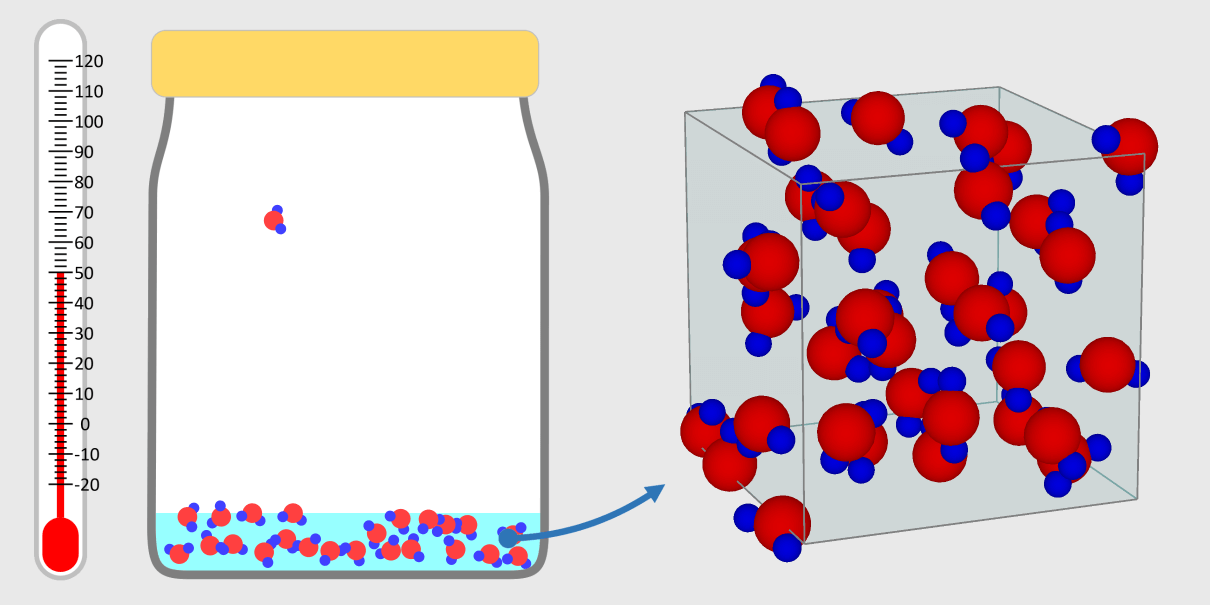
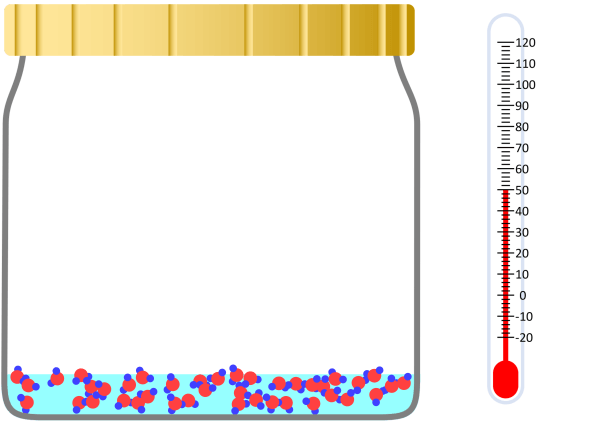
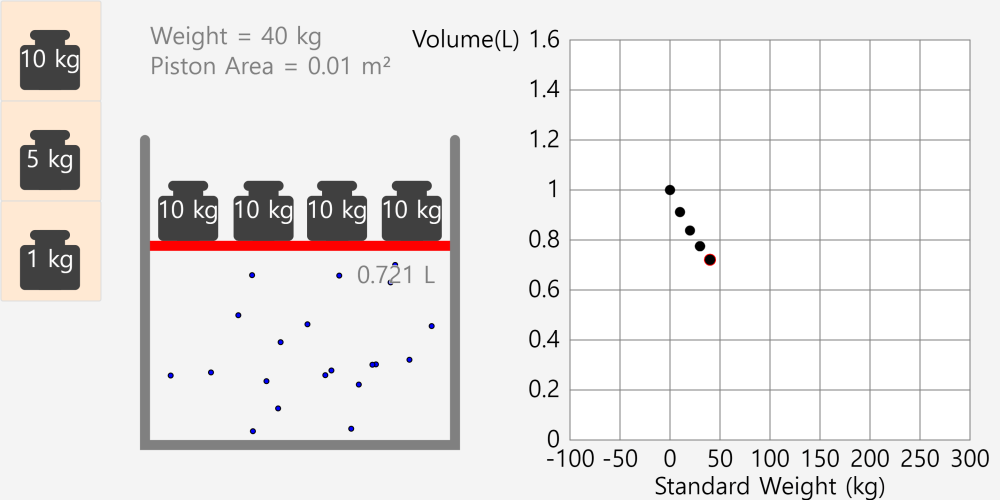
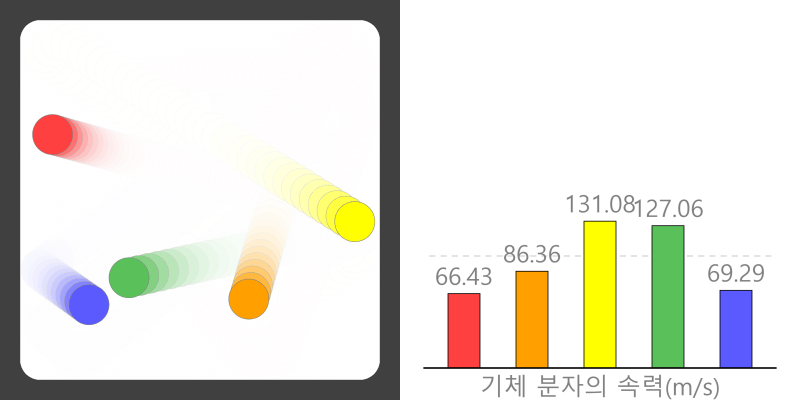
Kinetic theory model Gas particles are constantly moving in all directions. However, we cannot observe these gases because they are very small. The “Kinetic theory model” can replace these invisible gas particles with beads and make … more