- A solid metal can be made by grouping several hot gaseous (monatomic) metals together.
- What is the difference between emission spectra in the gas (monatomic) and solid state?
- The simulation above is a simple example and is not representative of real atoms.
Line Spectrum
Atoms can absorb incoming energy and radiate that energy back to their surroundings. The energy emitted by a single atom, such as a monatomic molecule or a metal gas, can only have definite values. This is because the electrons bound to the nucleus can only have quantized energy.
Continuous Spectrum
If there is only one atomic nucleus, the explanation will end as above, but if atomic nuclei are densely attached, such as in a solid, the electron orbits of the atoms overlap each other and become thicker.
As a result, the energy level now takes the form of a band with a certain width rather than a single line.
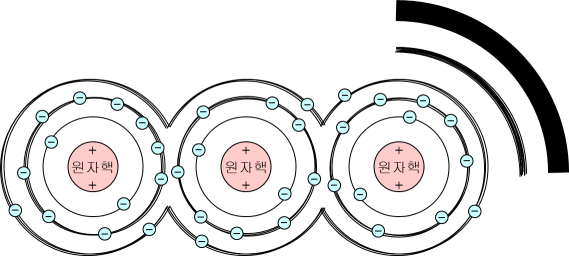
These thickened energy levels are called energy bands. And, since electrons cannot exist between bands and bands, and between lines and bands, it is called the energy gap.
When a band is formed, the emitted energy also varies, and some continuous spectrum begins to be observed.
Example: Low-Pressure Sodium Lamp (LPS Lamp) and High-Pressure Sodium Lamp (HPS Lamp)
A sodium lamp is a device that emits bright light by discharging a small amount of sodium inside a vacuum discharge tube.
Sodium lamps can be divided into Low-Pressure Sodium(LPS) Lamps and High-Pressure Sodium(HPS) Lamps according to the gas density inside.
Because the low-pressure sodium lamp is close to the metal gas, It emits about 590mm wavelength light. (Same as sodium flame reaction color)
On the other hand, high-pressure sodium lamps form a continuous spectrum due to the mutual interference of atoms and become closer to white.
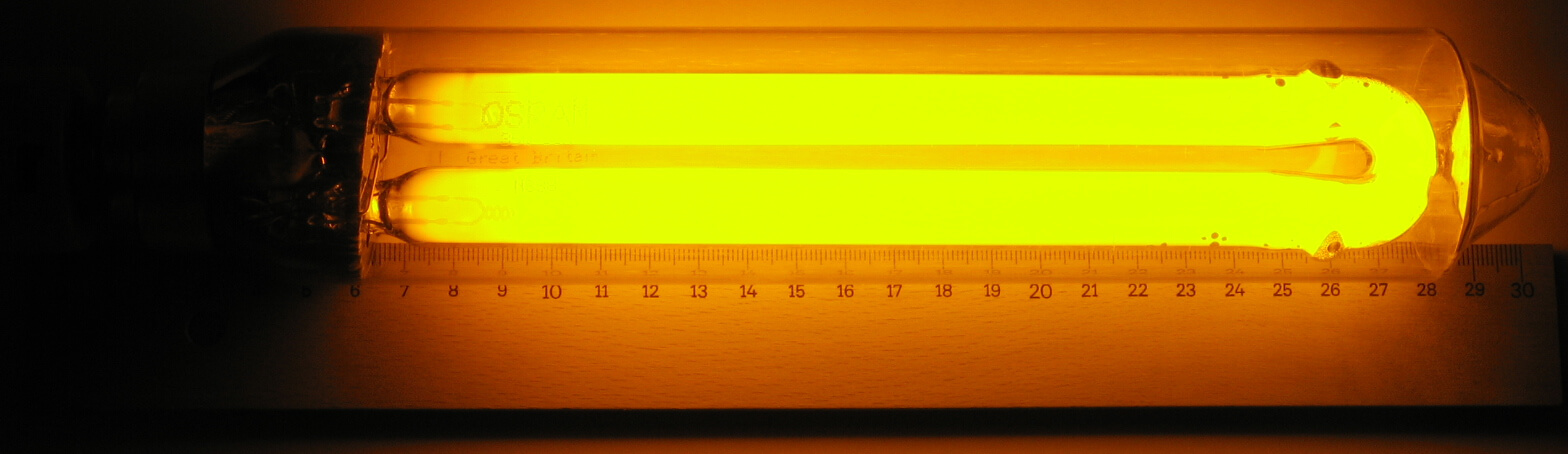
Low-Pressure Sodium Lamp. (2023, August 19). In Wikipedia. https://en.wikipedia.org/wiki/Sodium-vapor_lamp

High-Pressure Sodium Lamp. (2023, August 19). In Wikipedia. https://en.wikipedia.org/wiki/Sodium-vapor_lamp
Why are most solid metals silvery?
Most metals have large electronic bands outside their atomic structure (metal bonds). And free electrons can move freely in the band. Because of this, metals can absorb most wavelengths of visible light and re-emit them as is.