Heat and molecular motion
Every object is made up of atoms or molecules that are small particles that the eye can not see. These particles are constantly moved or oscillated themselves. This is called molecular motion.
The higher the molecular motion, the higher the temperature. The slower the molecular motion, the lower the temperature. Thus, the temperature is a physical quantity that shows the activity level of molecular motion.
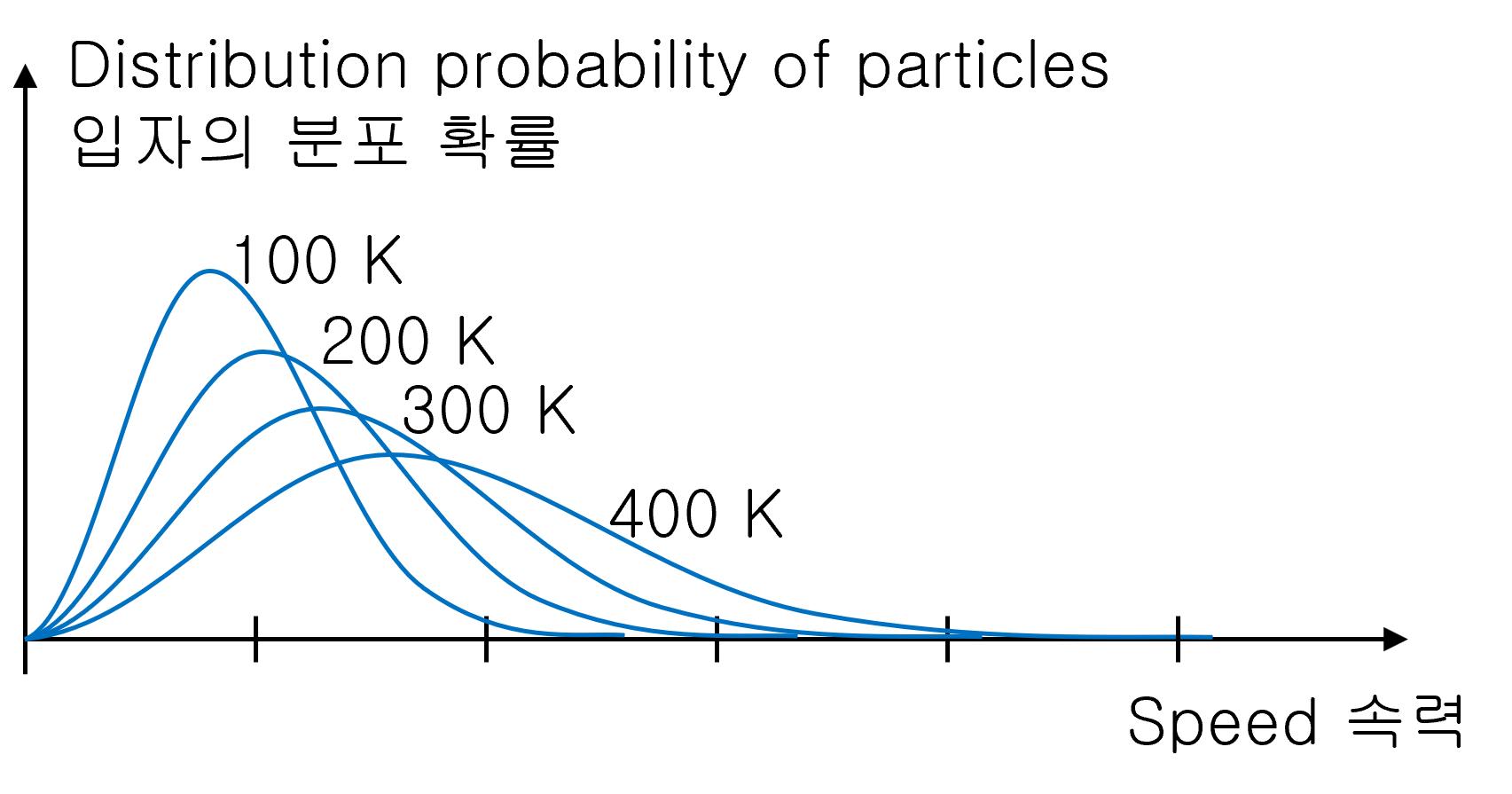
Distribution of particle’s velocity with temperature
Low-temperature gas is mainly distributed on the slower side.
As the temperature of the gas increases, the graph becomes more widespread. And the average speed of the gas also increases.
Why does the chemical reaction get fast when the high temperature?
For chemical reactions to proceed, atoms or molecules must collide at a sufficient speed. The higher the temperature, the more gas reaches the minimum collision speed required for a chemical reaction. Therefore, the higher the temperature, the better the chemical reaction.
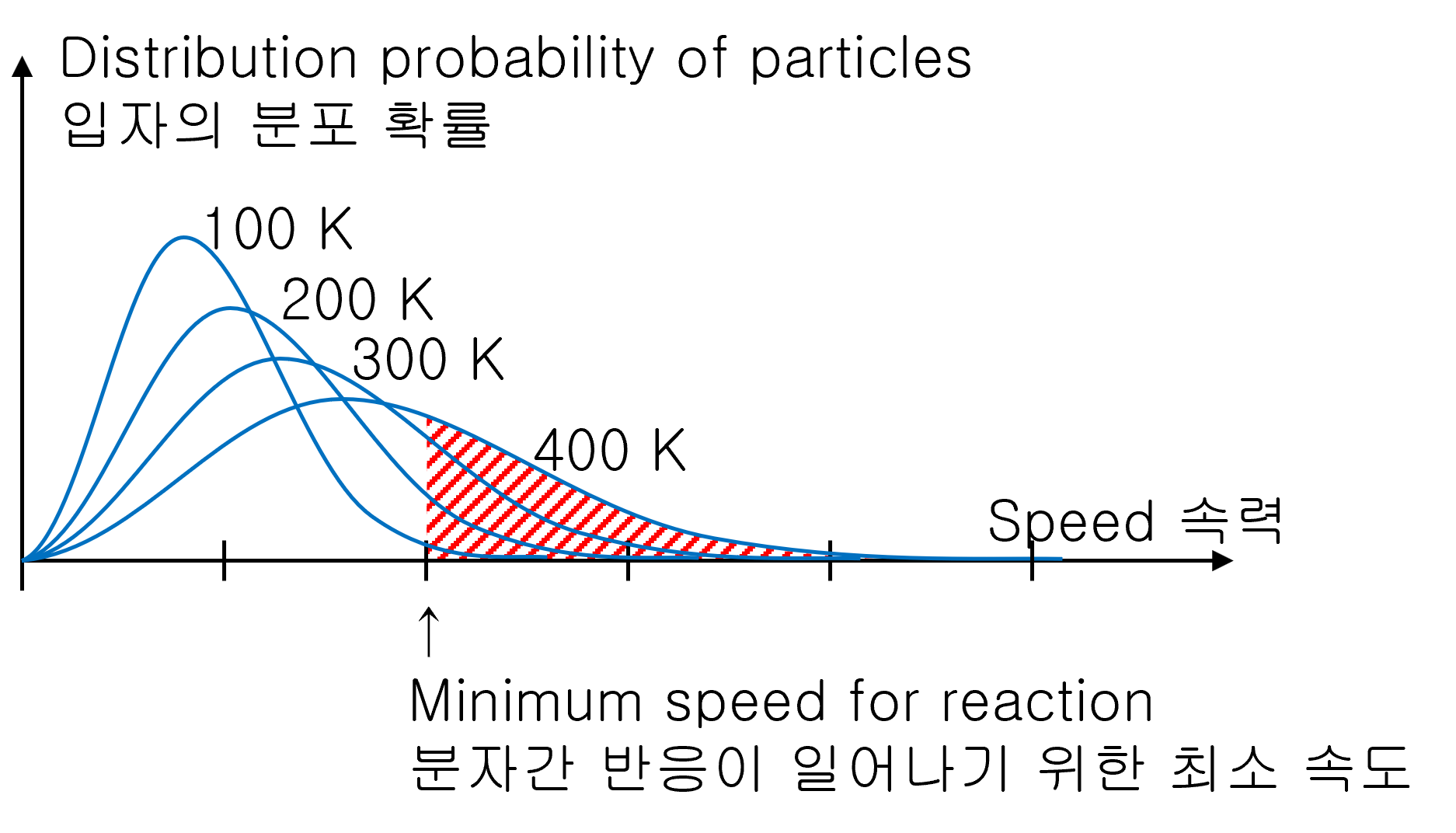
And, the low temperature does not mean that the reaction does not occur. Although there is a low probability, there is always the possibility of encountering molecules at high speeds. Therefore, the chemical reaction proceeds little by little.
For example, even if the water is not heated to 100°C, some water molecules can reach enough speed to escape water vapor. Some water particles can jump from the water surface to the atmosphere. This is called evaporation.