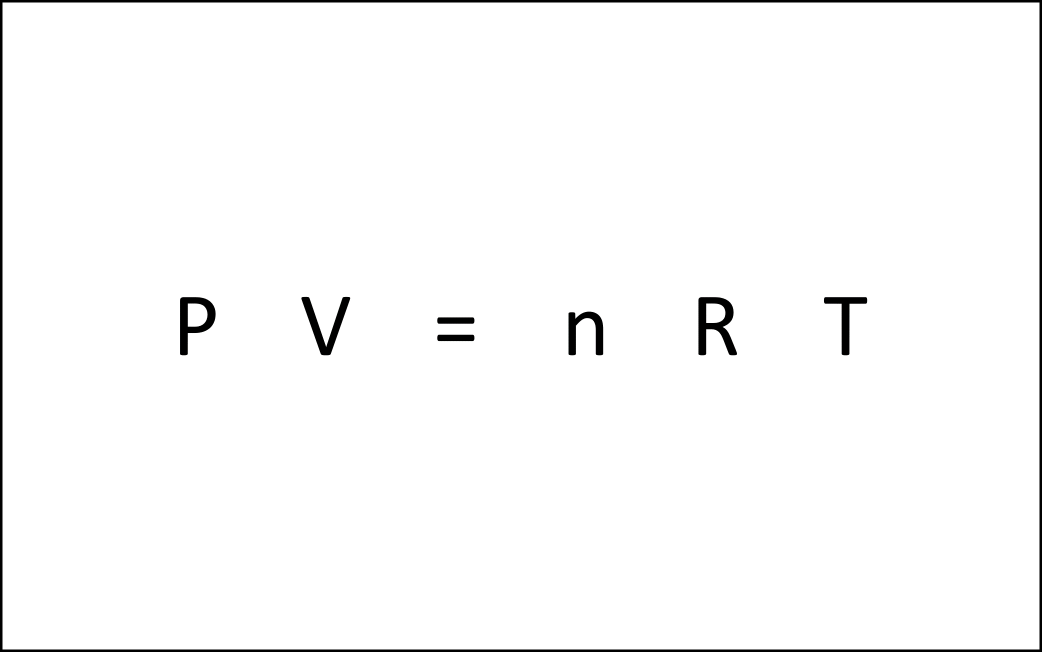Kinetic Molecular Model of Gases
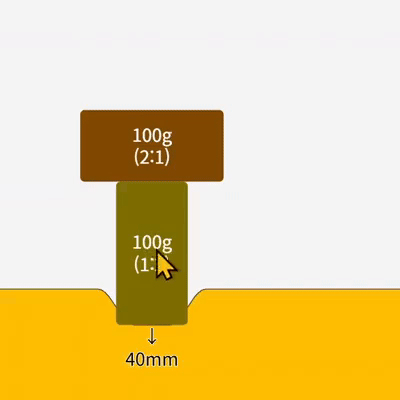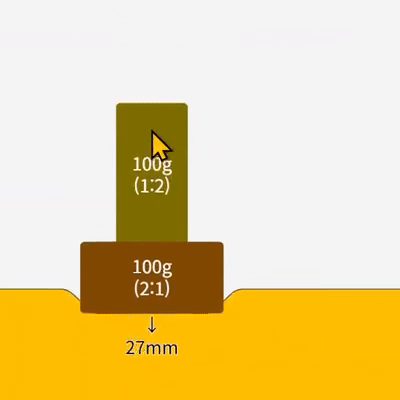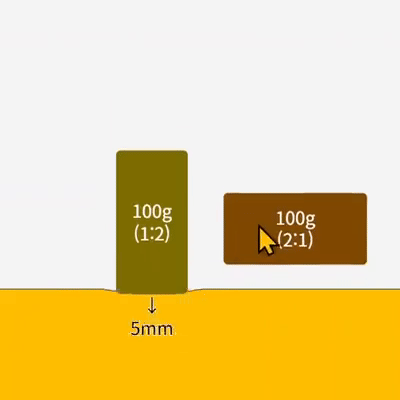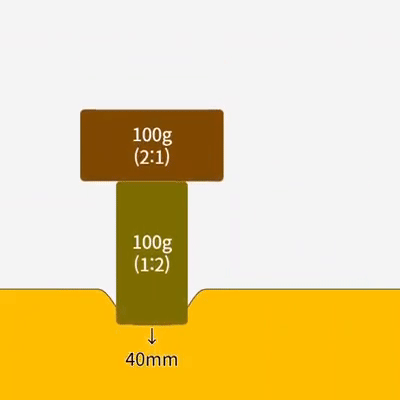
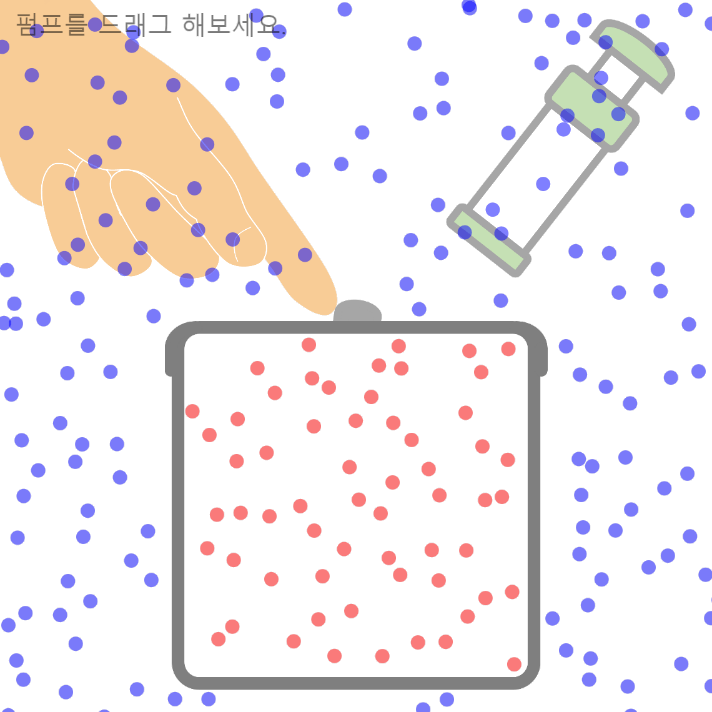
Characteristics of Gases Unlike solids and liquids, gases have the following unique characteristics: 1. They have no definite shape or volume Gas particles move in all directions and can fill any part of a container. Therefore, … more