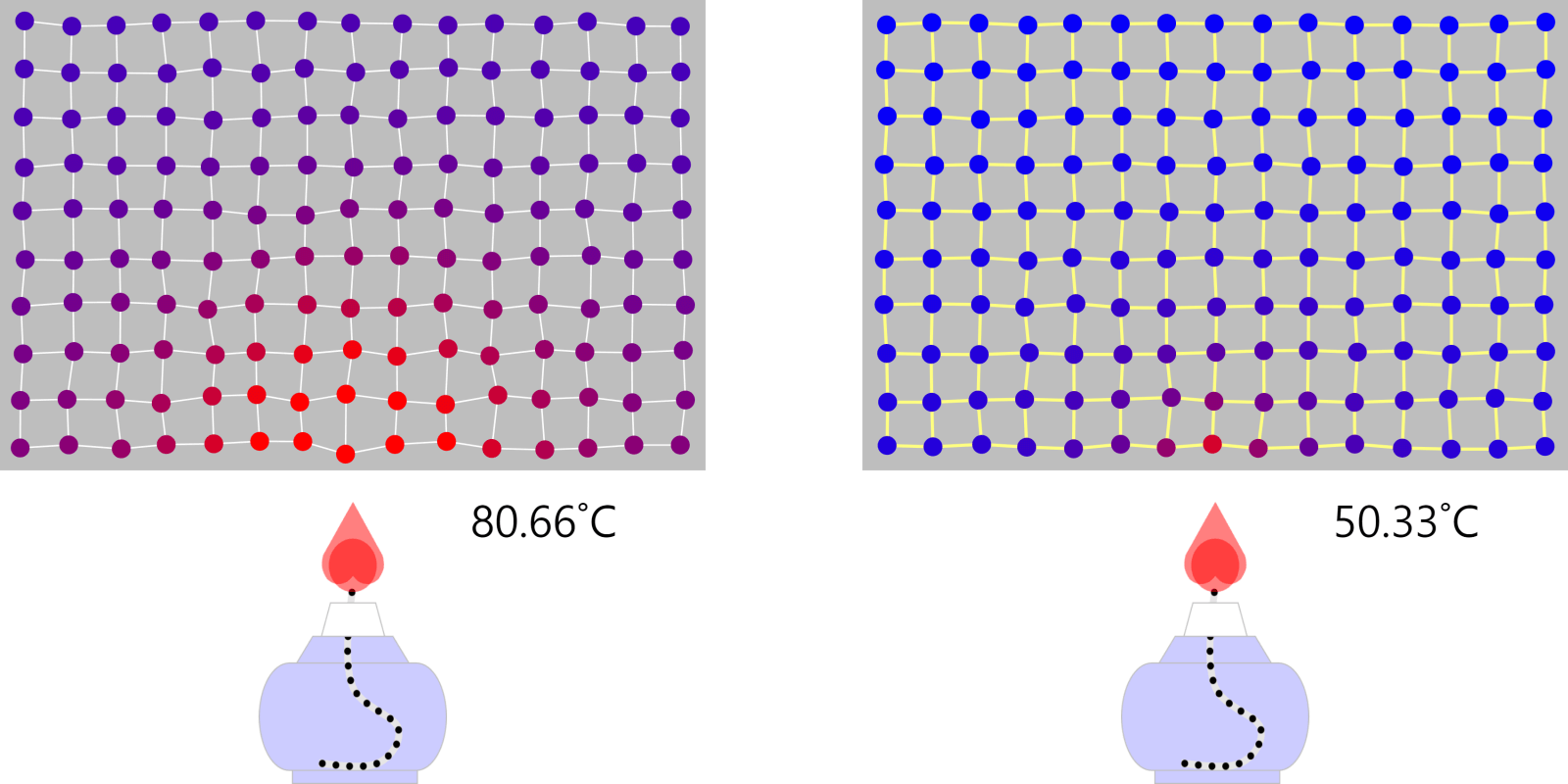
Heat conduction (a good thing about wooden pot handles)
Conduction The ‘conduction’ of heat is how heat is transferred by moving particles that make up an object to neighboring particles. Heat can be transferred even if the particles do not move, mainly by vibration or … more