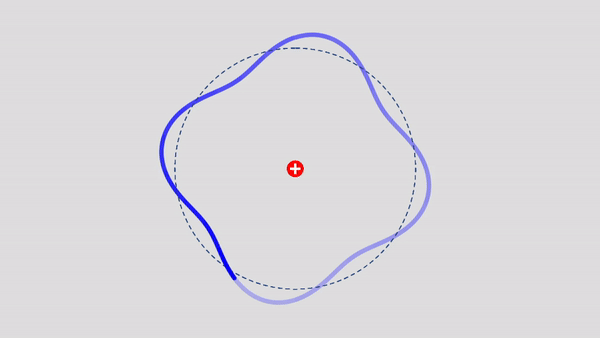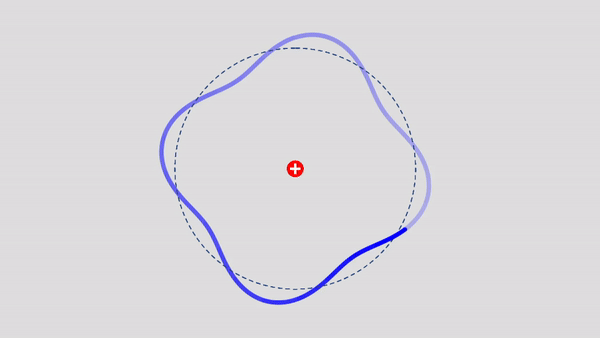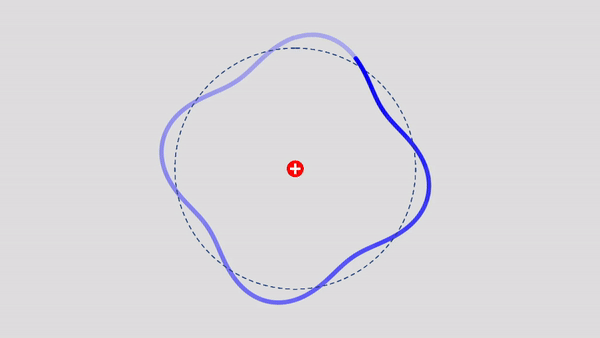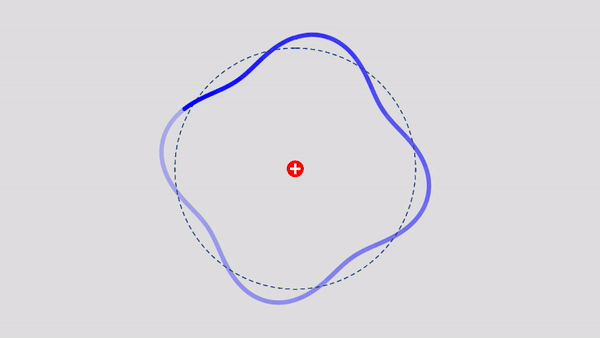
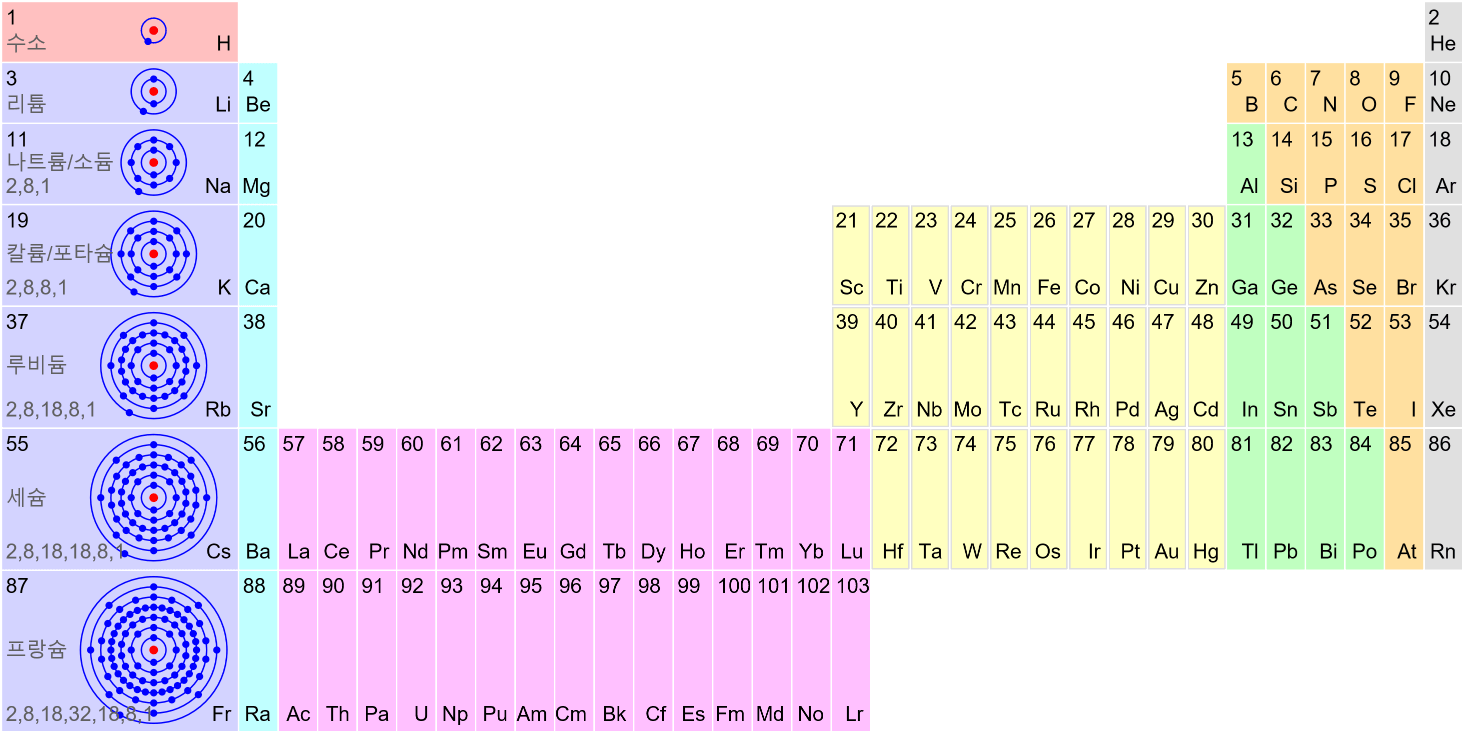Matter Wave and de Broglie’s Atomic Model
Light has both particle and waves properties at the same time. Likewise, de Broglie (France, 1892 – 1987) thought that such things as electrons that had so far been considered particles might possess wave nature (1932). … more