* This simulation take the spectral data from NATIONAL INSTITUTE OF STANDARDS AND TECHNOLOGY.
When heat is applied to the atoms, some electrons can have high energy levels and fall to the lower levels. Some electrons emit as much energy as the energy level difference. By measuring the emitted light, we can detect the atoms.
The metal atoms mainly emit visible light. Metal-containing ionic material with gunpowder creates a fantastic fireworks display.
Continuous spectrum
Incandescent bulbs emit light in all visible wavelengths by thermoelectrons. The spectrum’s continuous view is not only because a specific value of energy is emitted but because energy is evenly emitted over the entire visible range.
The continuous spectrum is well observed in a dense state because the atom’s electron orbits interfered with each other and thus did not emit their own light. (E.g., solid or liquid, dense gas)
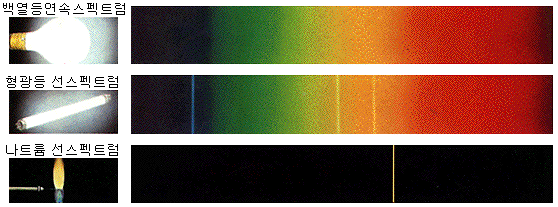
Line spectrum
When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light appear. The observed spectrum looks like a bar code.
Line spectra are well observed in lean conditions because the atoms must emit their own light without interfering with each other. (E.g., gas or plasma state)