What is Covalent Bond?
The covalent bond is a bond formed by atoms. Each atom creates an electron pair and shares it. Because each other's electrons are shared in pairs, the number of electrons involved in sharing is always even.
The electron pair that participates in the covalent bond is called the 'shared electron pair,' and the electron pair that does not participate in the covalent bond is called the 'unshared electron pair.'
It is divided into 'single bond,' 'double bond,' and 'triple bond' depending on the number of electron pairs shared between two atoms.
Single Bond
Two atoms share one electron pair (or two electrons).
A single line between atoms represents it.
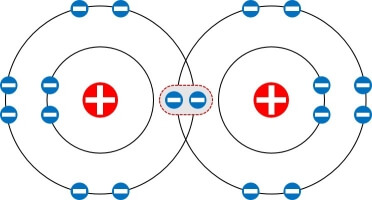
F-F (Fluorine molecule)
Double Bond
Two atoms share two electron pairs (or four electrons).
Double lines between atoms represent it.
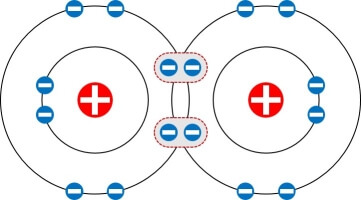
O=O (Oxygen molecule)
Triple Bond
The two atoms share three electron pairs (or six electrons).
A triple line between atoms represents it.
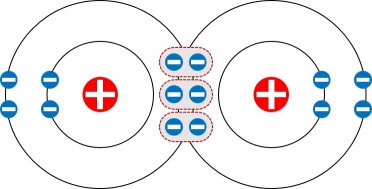
N≡N (Nitrogen molecule)