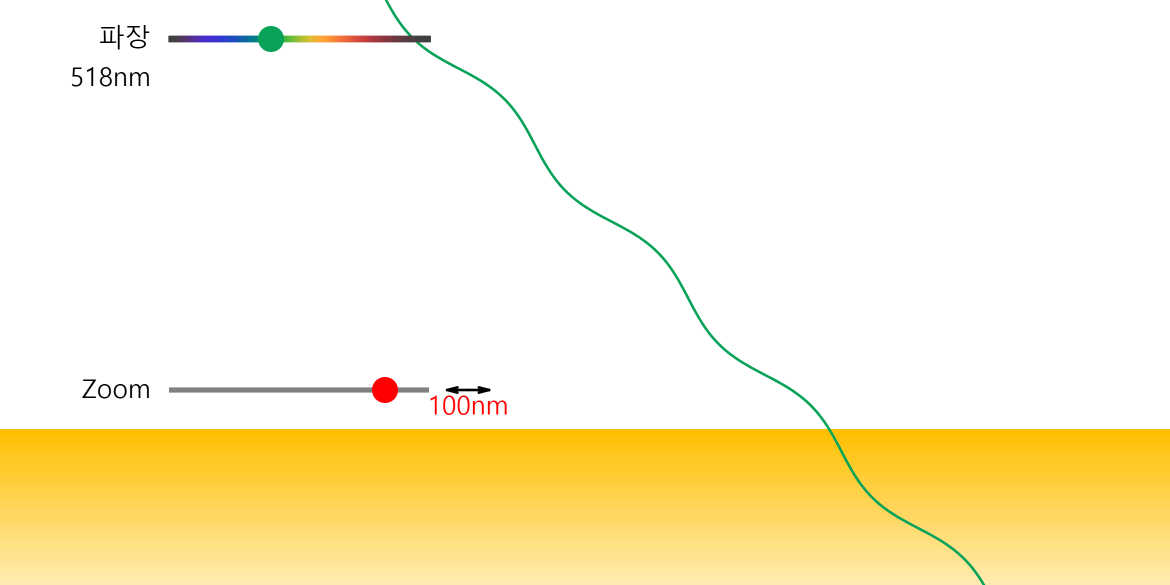
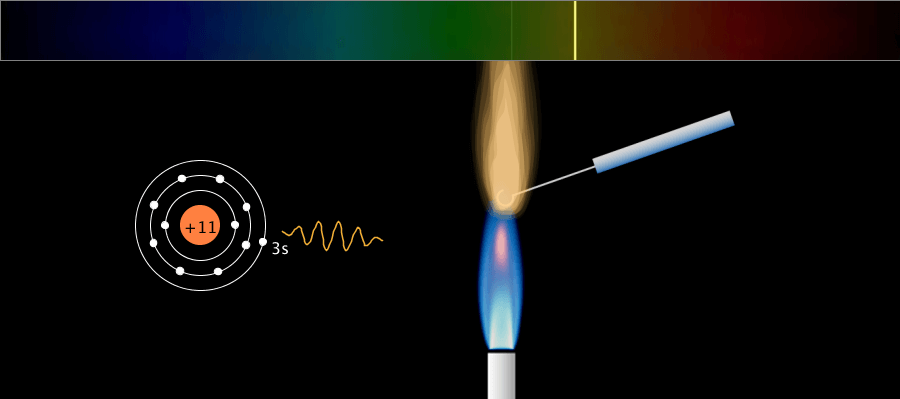Line Spectrum and Continuous Spectrum
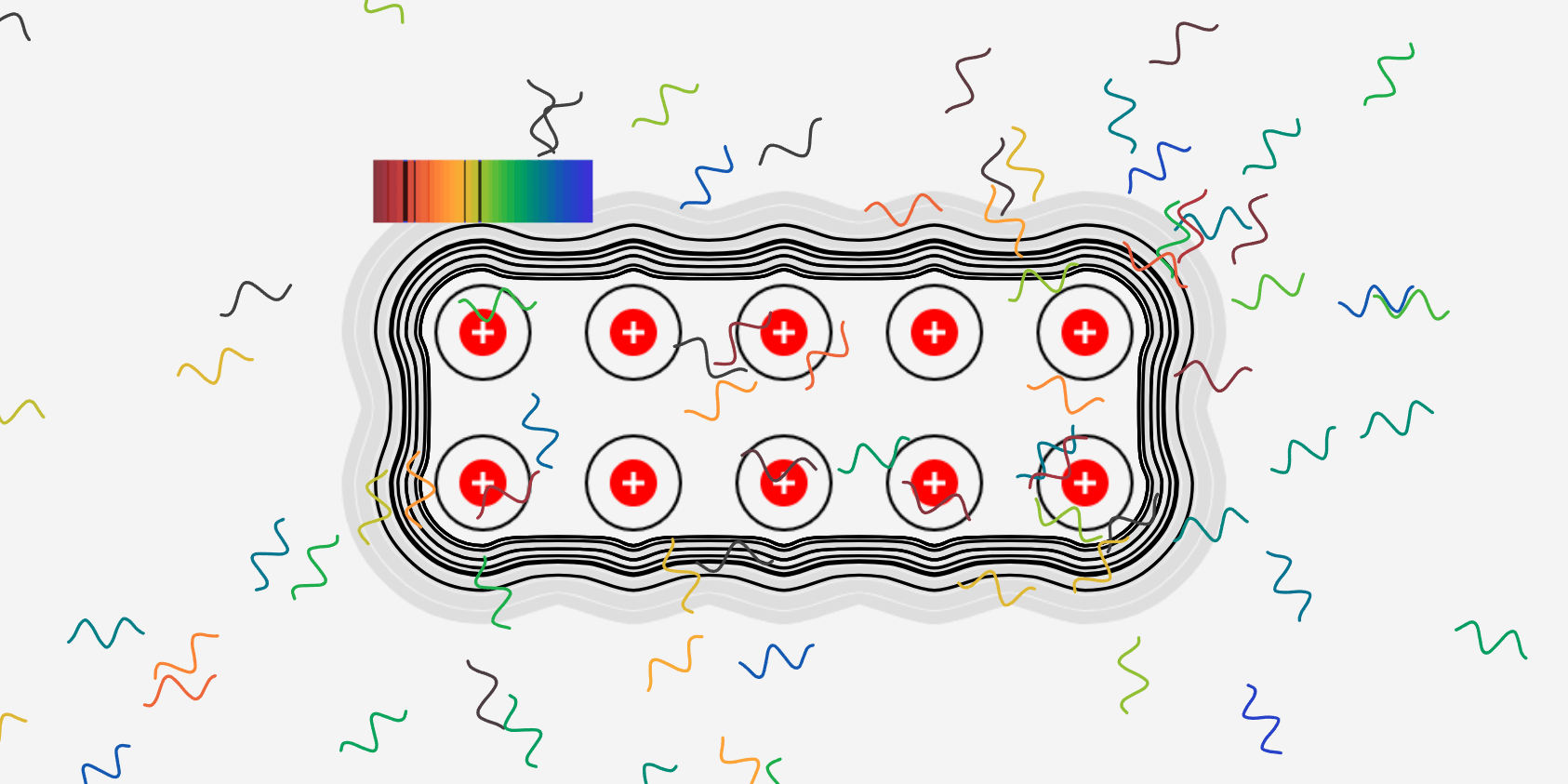
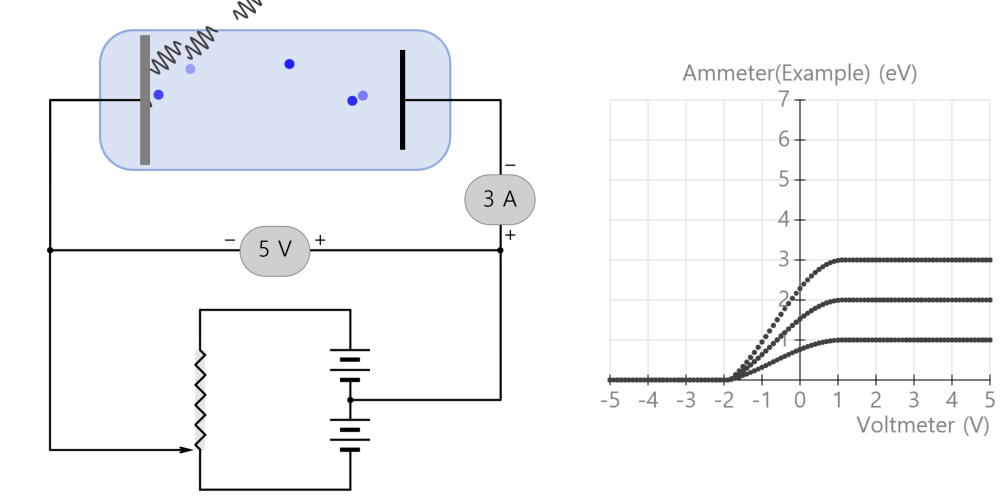
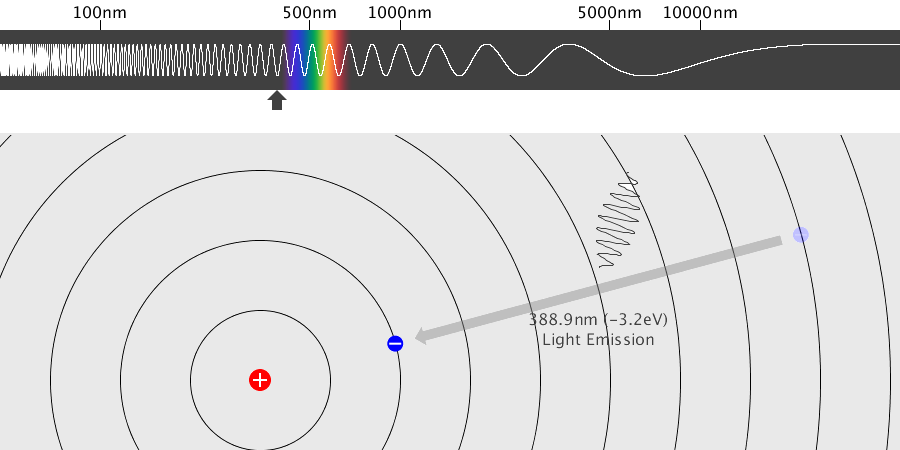
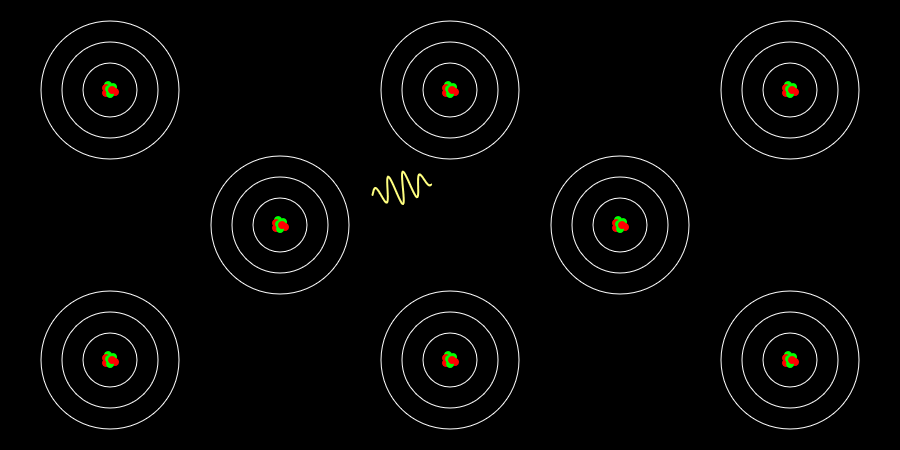
A solid metal can be made by grouping several hot gaseous (monatomic) metals together. What is the difference between emission spectra in the gas (monatomic) and solid state? The simulation above is a simple example and … more