A heat engine
A heat engine is a machine that moves by moving energy from a hot space to a cold space. In this process, the heat engine converts some of the heat energy into mechanical energy.
All heat engines return to their original state after a series of conditions. During this cycle, a heat engine gives some energy to the outside.
Like a car's engine, all the machines that move using thermal energy are heat engines.
Some heat engines operate reversibly like air conditioners. In other words, the air conditioner moves heat energy because it receives external work.
A Carnot engine
A Carnot engine is a type of heat engine based on a reversible Carnot cycle.
A Carnot engine consists of the following three parts.
1. Cylinder with monatomic gas
2. Piston that can move freely without friction
3. Free part for heating and cooling
The internal gas can expand and give some work(mechanical energy) to the outside. Conversely, it can be compressed from the outside.
The amount of work whenever gas expands is greater than compress. Therefore, A Carnot engine works well as a kind of engine.
The operating cycle of the Carnot engine
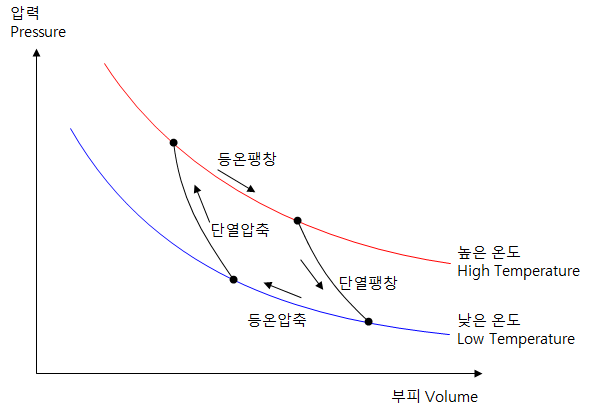
- Isothermal expansion - The gas absorbs heat from the cylinder, which is kept at a high temperature.
- Adiabatic expansion - The temperature is lowered as the gas expands.
- Isothermal compression - the gas discards heat from the cylinder that is kept at low temperature.
- Adiabatic Compression - The temperature rises as the gas compresses.
The meaning of the Carnot engine
Carnot engine is a type of ideal heat engine, and this engine does not actually exist. Even if each process is reversible, it becomes an irreversible process due to the operation's friction. Also, the process of adiabatic expansion and adiabatic compression can not actually occur. This is because there is no material that completely blocks heat from the outside.
After all, the Carnot engine's meaning is that it is the most efficient heat engine logically possible.
Efficiency of the heat engine
To convert heat energy to 100% mechanical energy, the heat must be thrown at a temperature of zero (0K). But such low temperatures are not really possible.
Even if the Carnot engine is theoretically the most efficient heat engine, it can not convert all thermal energy into mechanical energy. Therefore, the efficiency of a thermal engine is usually lower.