- Press the ‘C’ button to add carbon, press the ‘H’ button to add a hydrogen atom, and press the ‘O’ button to add an oxygen atom.
- The added atoms are randomly placed and may not give the correct shape.
- You can create molecules with up to 30 carbons.
- You can move the position of the atom.You can only create a single join in this simulation.
Alkanes
Alkanes are chemical compounds that consist only of the elements carbon and hydrogen linked exclusively by single bonds.
Each carbon atom forms four bonds (either C-H or C-C bonds). Each hydrogen atom is connected to a single carbon atom by an H-C bond.
The alkanes have two primary commercial sources: petroleum and natural gas.
Saturated oils and waxes are examples of larger alkanes in which the number of carbon atoms in the carbon chain is greater than 10.
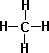
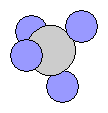
- Methane CH4
- Ethane C2H6
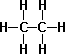
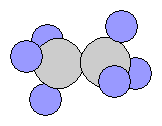
- Propane C3H8
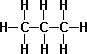
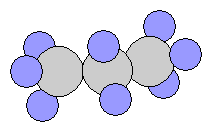
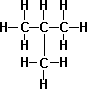
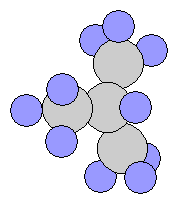
- n-Butane C4H10
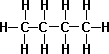
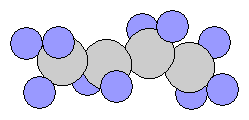
- iso-Butane(Isomer of Butane) – It is a compound with the same chemical formula but internally different structure, slightly different chemical properties such as melting point and boiling point.