The energy level of the atom
Atoms are made up of an atomic nucleus and electrons that revolve around the nucleus. In Bohr's atomic model, electrons can change their position by receiving energy (mainly light energy). But what's unique is that electrons can only stay in special locations.
Atomic light absorption and emission
The atom absorbs the incoming energy and releases that energy back to its surroundings in the following order.
1. Electrons at a low level absorb light energy and can be lifted into a high state.
2. Electrons whose energy is increased by receiving moderate light energy rise to a high level.
3. Electrons that have risen to a high level generally do not last long (about 1 billionth of a second) and come down to their original position. At this time, they emit light energy that they absorbed again.
The time it takes for an electron to travel between energy levels is very short. This is called the 'quantum leap' because it moves the energy level instantaneously in a very short time.
How can I quantitatively explain why light is slow in an object with a large refractive index?
The light incident on the glass causes the molecules to vibrate and is continuously absorbed and re-emitted.
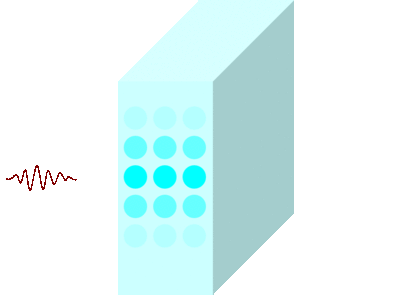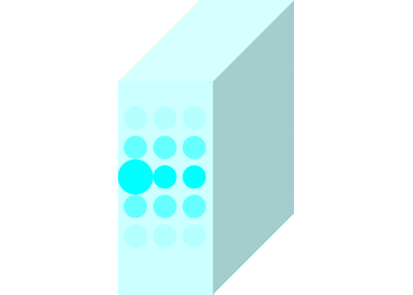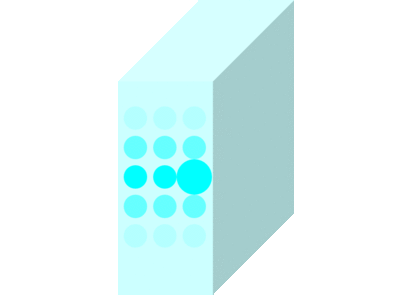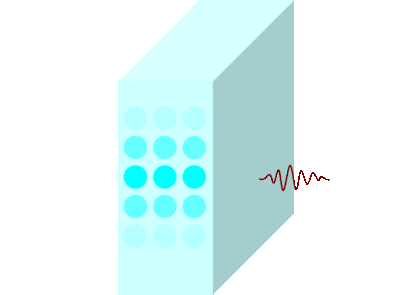
Light passes through matters in this way and travels to the other side.
Light travels slowly in matters due to the time delay between absorption and re-emission.